Abstract
Background: The autoimmune disorder immune thrombocytopenia (ITP) is characterized by immune-mediated platelet destruction and impaired production, leading to a predisposition to bleeding and diminished quality of life. Although initially responsive to standard therapy, most patients do not attain durable remission or are challenged by long-term tolerability. Rilzabrutinib is the first oral, reversible Bruton tyrosine kinase (BTK) inhibitor that targets mechanisms driving ITP without effects on normal platelet aggregation. Preclinical studies showed simultaneous rapid anti-inflammatory effects of rilzabrutinib and neutralization and prevention of autoantibody signaling (Langrish J Immunol 2021). In an ongoing phase I/II study, the optimal dose of 400 mg bid rilzabrutinib (± concomitant ITP therapy) was well tolerated and resulted in rapid and durable platelet response across several subgroups of patients and with extended treatment.
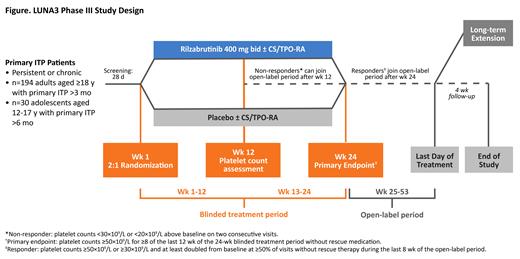
Study Design and Methods: LUNA3 is the first randomized, multicenter, phase III study evaluating the efficacy and safety of rilzabrutinib vs placebo with open-label extension in adult and adolescent patients with persistent or chronic ITP (NCT04562766; EudraCT 2020-002063-60). Eligible patients must have primary ITP for >6 months if aged 12-17 years or >3 months if aged ≥18 years and an average of 2 platelet counts <30×10 9/L (no single count >35×10 9/L) within 2 weeks prior to study treatment. Additionally, patients should have a previous response (platelet count ≥50×10 9/L) to corticosteroids or intravenous immunoglobulin/anti-D that was insufficient or not sustained, or documented intolerance or insufficient response to any appropriate courses of standard-of-care ITP therapy. Randomization is carried out separately for the 2 age groups (12-17 years and ≥18 years), and then patients are stratified by splenectomy status (yes/no) and thrombocytopenia severity (platelet counts <15×10 9/L or ≥15×10 9/L; Figure). Patients are randomized 2:1 to oral rilzabrutinib 400 mg bid or placebo to receive double-blind treatment for up to 24 weeks, followed by open-label treatment for 28 weeks, and then a 4-week safety follow-up. After the 12-week double-blind treatment, patients who are no longer responding (ie, platelet counts <30×10 9/L or <20×10 9/L above baseline on two consecutive visits) have the option to receive open-label rilzabrutinib or discontinue from the study. Concomitant stable doses of ITP medication (corticosteroids and/or thrombopoietin receptor agonists) are permitted if they were maintained for ≥2 weeks prior to study day 1. Patients who receive rescue medication are allowed to continue study treatment. The primary endpoint is the achievement of platelet counts ≥50×10 9/L for ≥8 of the last 12 weeks of the 24-week blinded treatment period in the absence of rescue medication. Secondary endpoints include safety, duration of and time to platelet responses, rescue therapy use, and changes from baseline in ITP Bleeding Scale (IBLS) scores. Responders who had platelet counts ≥50×10 9/L or ≥30×10 9/L and at least doubled from baseline at ≥50% of visits without receiving rescue therapy during the last 8 weeks of the open-label period may enter an additional 12-month long-term extension. This clinical trial was initiated in 2020, with the planned enrollment goal of approximately 194 adult and 30 adolescent patients globally. The study results will provide further support for a unique treatment mechanism via BTK inhibition to target the underlying pathology of ITP.
Kuter: Platelet Disorder Support Association: Membership on an entity's Board of Directors or advisory committees; Up-to-Date: Patents & Royalties: Up-To-Date; Rubius: Current equity holder in publicly-traded company; Actelion (Syntimmune), Agios, Alnylam, Amgen, Argenx, BioCryst, Bristol Myers Squibb (BMS), Caremark, CRICO, Daiichi Sankyo, Dova, Genzyme, Immunovant, Incyte, Kyowa-Kirin, Merck Sharp Dohme, Momenta, Novartis, Pfizer, Principia, Protalex, Protalix, Rigel: Consultancy, Other: grant support and consulting fees; Actelion (Syntimmune), Agios, Alnylam, Amgen, Argenx, Bristol Myers Squibb (BMS), Immunovant, Kezar, Principia, Protalex, Rigel, Takeda (Bioverativ), UCB: Research Funding. Bussel: CSL: Other: DSMB; UCB: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: DSMB; Dova/Sobi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; UptoDate: Honoraria; RallyBio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; Argenx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Principia/Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Momenta/Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees. Cooper: Principia and Sanofi: Consultancy; Sanofi and Principia: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel, accommodations expenses. Gernsheimer: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: personal fees ; Amgen: Honoraria; Cellphire: Consultancy; Dova: Consultancy, Honoraria; Principia: Membership on an entity's Board of Directors or advisory committees, Research Funding; Rigel: Membership on an entity's Board of Directors or advisory committees, Research Funding; NHLBI: Research Funding. Lambert: Dova: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Rigel: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Shionogi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Principia: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; PDSA: Research Funding; ClinGen, ISTH, ASH, GW University: Honoraria; Argenx: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sysmex: Research Funding; Bayer: Consultancy; Octapharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Liebman: Sanofi, Momenta: Research Funding; Alexion, Novartis, Dova: Consultancy. Tarantino: Grifols: Research Funding, Speakers Bureau; Novo Nordisk: Research Funding; Pfizer: Research Funding; Principia: Membership on an entity's Board of Directors or advisory committees, Research Funding; Spark Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; UCB: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dova: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Octapharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sobi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BioMarin: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees. Bandman: Sanofi: Ended employment in the past 24 months. Arora: Principia Biopharma Inc, a Sanofi Company: Ended employment in the past 24 months. Neale: Principia Biopharma: Divested equity in a private or publicly-traded company in the past 24 months; Principia Biopharma/Sanofi: Ended employment in the past 24 months. Burns: Sanofi: Ended employment in the past 24 months. Yao: Sanofi: Current Employment. Ghanima: Amgen, Novartis, Pfizer, Principia Biopharma Inc- a Sanofi Company, Sanofi, SOBI, Griffols, UCB, Argenx: Consultancy; Bayer, BMS/Pfizer: Research Funding; Amgen, Novartis, Pfizer, Bristol Myers Squibb, SOBI, Griffols, Sanofi: Honoraria.
Rilzabrutinib is an investigational therapy being evaluated in a clinical study for the treatment of patients with immune thrombocytopenia.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal